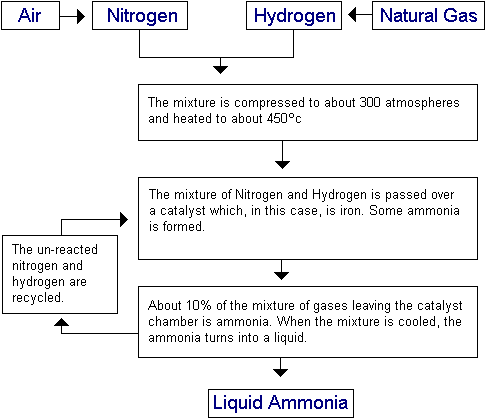
The Haber process is the process by which Ammonia is produced. Simply put, it involves combining Nitrogen and Hydrogen together as the second equation below shows. This is a reversible reaction.
The nitrogen needed for the reaction comes from the fractional distillation of liquid air. The hydrogen comes from natural gas (methane – CH4). This is reacted with steam to produce hydrogen and carbon monoxide, as shown in the first equation below. The carbon monoxide is removed after this, to leave the needed hydrogen.
Ammonia has many uses:
- Making carpets
- Modern fabrics for clothes
- Ammonia fertilizers
- NH4NO3 (ammonium nitrate) is also used as a fertilizer in the form of granules
The reactions involved are as follows. The second equation is a reversible reaction:
- CH4 (g) + H2O (l) —-> CO (g) + 3H2 (g)
- N2 (g) + 3H2 (g) <—-> 2NH3 (g) [Ammonia]