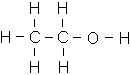Alcohols make good fuels because they burn easily and they release a lot of heat energy, which can be harnessed to power machines etc…
The combustion of alcohol is an exothermic reaction and this means that heat is given out from the reaction, opposed to being taken in, which would occur in an endothermic reaction. The following equation shows the result of combustion of alcohol (in this case, ethanol).
- Alcohol + Oxygen —-> Carbon Dioxide + Water
- C2H5OH + 3O2 —-> 2CO2 + 3H2O
The general formula for alcohols is as follows:
- CnH2n+1OH (where “n” is a constant)
The Alcohols form a homologous series where they all have similar chemical structures and properties. They are produced from either anaerobic respiration or by reacting water with the alkenes (Hydration).
The first five members of this homologous series are:
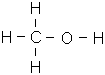
- CH3OH (Methanol)
- C2H5OH (Ethanol)
- C3H7OH (Propanol)
- C4H9OH (Butanol)
- C5H11OH (Pentanol)